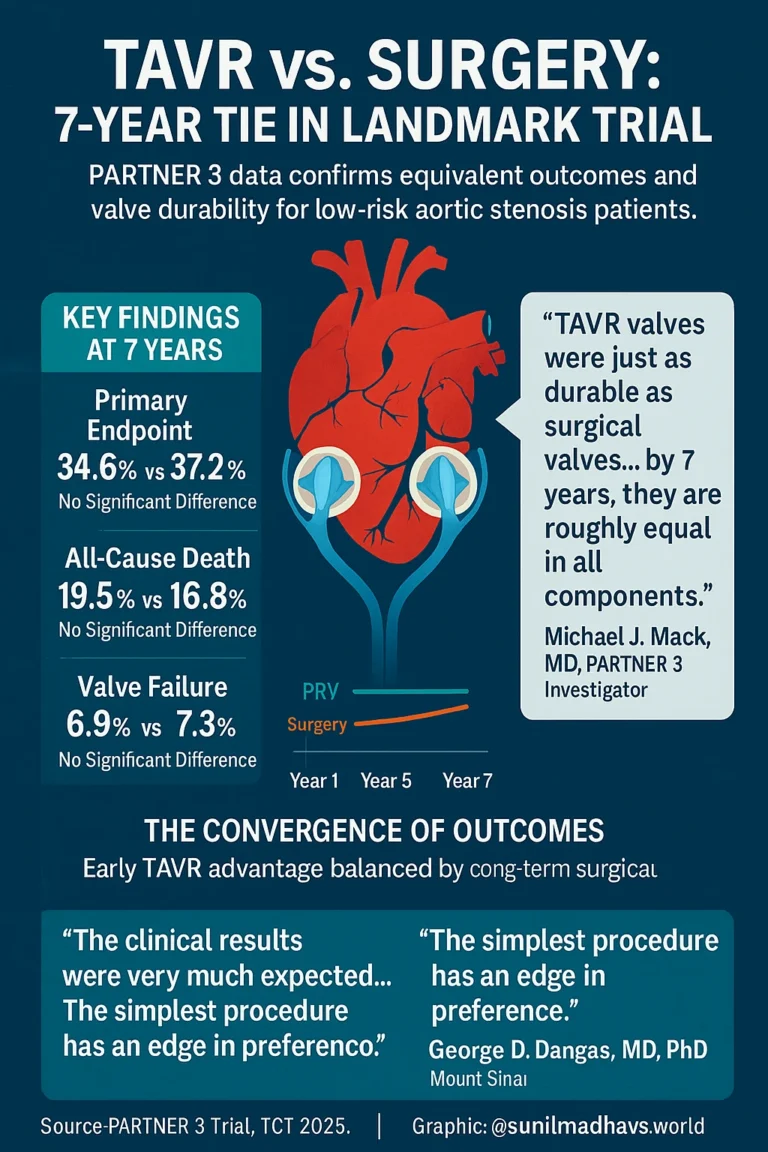
SAN FRANCISCO — Seven-year follow-up data from the landmark PARTNER 3 trial have confirmed that transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) offer comparable long-term outcomes and valve durability in patients with severe aortic stenosis who are at low surgical risk.
The results, presented by Michael J. Mack, MD, at the TCT 2025 Scientific Symposium, reinforce that the early advantages of TAVR are balanced by surgery over time — resulting in nearly identical clinical outcomes by year seven.
“At 7 years, there was no difference in clinical outcomes or valve durability between transcatheter and surgical aortic valve replacement,” Mack said.
“TAVR valves were just as durable as surgical valves.”
Background: A Decade of Data Comes Full Circle
The PARTNER 3 trial, the first randomized study to compare TAVR using the balloon-expandable Sapien 3 valve (Edwards Lifesciences) with traditional surgery in low-risk patients, initially demonstrated TAVR’s superiority at one year. Patients receiving TAVR had fewer deaths, strokes, and rehospitalizations than those undergoing open-heart surgery.
By five years, however, those early differences had disappeared. The new seven-year analysis presented by Dr. Mack confirms that both procedures now perform equally well in terms of patient survival, stroke rates, rehospitalization, and valve durability.
“What is very clear is that … TAVR won the first year, no question about it, but surgery wins after that,” Mack said.
“There is catch-up occurring with surgery so that by 7 years, they are roughly equal in all components.”
Study Design and Patient Population
The trial enrolled 1,000 patients (average age: 73 years) with severe symptomatic aortic stenosis who were classified as low surgical risk.
At 7 years, 92.7% of patients in the TAVR arm and 86.1% in the surgery arm were available for clinical follow-up. A vital status sweep extended mortality data to 95% and 93.8% of patients, respectively.
The primary composite endpoint—all-cause death, stroke, and rehospitalization—occurred in 34.6% of TAVR patients and 37.2% of surgical patients (HR = 0.87; 95% CI, 0.7–1.08; P = .21).
Hierarchical Win Ratio Analysis
The second primary endpoint, a hierarchical analysis combining all-cause death (including vital status data), disabling stroke, non-disabling stroke, and hospitalization days, demonstrated similar outcomes between TAVR and surgery.
- TAVR wins: 28.8%
- Surgery wins: 27.6%
- Ties: 43.6%
(Win ratio = 1.04; 95% CI, 0.84–1.3; P = .7)
“The attenuation of the primary endpoint differences observed at 5 years continued through 7 years,” Mack explained.
“In other words, the lines got closer together.”
Mortality, Stroke, and Valve Durability
At 7 years, there were no statistically significant differences between the TAVR and surgical groups in:
| Outcome | TAVR | Surgery | HR (95% CI) | P-value |
|---|---|---|---|---|
| All-cause death | 19.5% | 16.8% | 1.17 (0.86–1.59) | .31 |
| Bioprosthetic valve failure | 6.9% | 7.3% | 0.93 (0.53–1.56) | .78 |
| Aortic valve reintervention | 6.7% | 6.0% | 1.11 (0.63–1.94) | .72 |
Quality-of-life outcomes were also consistent between both procedures.
Among surviving patients:
- Kansas City Cardiomyopathy Questionnaire (KCCQ) score > 75:
– TAVR: 59%
– Surgery: 63.3% (P = .26) - Alive with no bioprosthetic valve failure:
– TAVR: 73.4%
– Surgery: 75% (P = .63)
“The marked 1-year improvement in hemodynamics and patient-reported outcomes were maintained through 7 years and similar for both therapies,” Mack said.
“Valve function and durability were excellent and similar in both groups.”
Expert Perspective
George D. Dangas, MD, PhD, MACC, MSCAI, professor of cardiology at the Icahn School of Medicine at Mount Sinai, said the results were consistent with real-world experience.
“The clinical results were very much expected,” Dangas explained. “They reflect what we see in practice — reassuring, with no surprises.”
He added that while hemodynamics and durability findings were anticipated, having long-term evidence is crucial.
“There was a lot of discussion because these kinds of long-term data were unknown,” he said. “It is important that this was figured out and described.”
Dangas emphasized that although both TAVR and surgical valve leaflets appear similar in quality, clinicians should be cautious about overgeneralizing the results.
“Extrapolation of these data to all sorts of valves would be a little premature,” he said.
“The technique has been studied for decades, but contemporary valves are different from historical ones, and some have not been well-studied at all.”
He stressed the importance of continuing long-term studies on newer-generation valves to understand durability trends more clearly.
“We need to continue these types of long-term hemodynamic and clinical studies on all types of valves, and to zero in on what we know and what we don’t know in terms of durability,” Dangas added. “But it looks like we haven’t gotten many things wrong so far.”
Dangas concluded that in low-risk patients, both procedures are strongly supported by current guidelines.
“There is a Class I recommendation for both procedures in this group,” he noted. “Ultimately, the decision comes down to a discussion between the referring doctor, the clinical cardiologist, the patient, and their family. The simplest procedure has an edge in preference — provided we can identify patients who are truly low risk for TAVR-related complications.”
Study Funding and Disclosures
The PARTNER 3 trial was funded by Edwards Lifesciences.
Dr. Michael J. Mack serves as co-principal investigator for trials sponsored by Abbott and Edwards Lifesciences, and as chair of a trial sponsored by Medtronic.
Dr. George D. Dangas reported no relevant financial disclosures.
Source:
Mack MJ, et al. Late-breaking clinical trials: Session II, in collaboration with the New England Journal of Medicine.
Presented at: TCT Scientific Symposium; Oct. 25–28, 2025; San Francisco (hybrid meeting).