Key Findings
- Brepocitinib demonstrated improvements in skin disease, muscle strength, and functional disability among patients with dermatomyositis.
- This marks the first successful phase 3 trial for a modern, targeted therapy in this condition.
- Once-daily brepocitinib showed benefits as early as 4 weeks, sustained for 1 year, while reducing reliance on steroids.
- Results come from the phase 3 VALOR study, evaluating brepocitinib (Priovant Therapeutics), an oral dual selective TYK2 and JAK1 inhibitor, presented at the Rheumatologic Dermatology Society annual meeting.
Background on Dermatomyositis
Dermatomyositis is a multiorgan inflammatory disease that causes severe muscle weakness and skin lesions, affecting about 40,000 adults in the U.S.. It can also lead to itchy rashes and impair organ function, especially in the lungs.
Anthony P. Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, explained:
“Dermatomyositis is a relatively uncommon autoimmune disease. Traditionally, the medications that we have used are immunosuppressive medications; first and foremost, systemic corticosteroids, and then the more traditional steroid-sparing immunosuppressive medicines, such as methotrexate, azathioprine, mycophenolate, mofetil and tacrolimus. For several decades, we have also known that intravenous immunoglobulin, or IVIG, can be a very good treatment for patients with dermatomyositis, regardless of whether we are trying to treat the inflammation in the skin or the muscles.”
Study Design
- Participants: 241 patients with definite or probable dermatomyositis.
- Groups: Brepocitinib 15 mg (n=81), brepocitinib 30 mg (n=81), placebo (n=79).
- Demographics: Mean age 50; over 70% women; over 70% on background corticosteroids.
- Steroid tapering: Between weeks 12–36, corticosteroids reduced to <5 mg/day, with further tapering at investigator discretion.
- Endpoints:
- Primary: Mean Total Improvement Score (TIS) at week 52 (30 mg vs placebo).
- Secondary: Disease activity across organ systems, quality of life, muscle strength, and skin outcomes.
Results
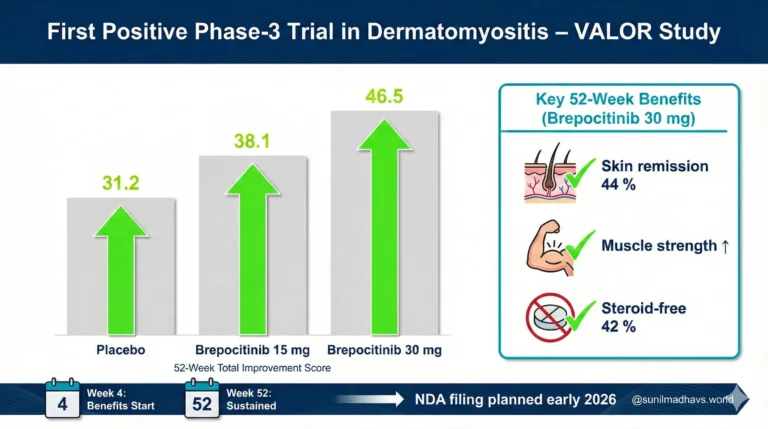
- TIS scores: 46.5 (30 mg group) vs 31.2 (placebo), difference of 15.3 (P = .0006).
- Response rates:
- Moderate response (TIS ≥40): 68% (30 mg) vs 44% (placebo), P = .004.
- Major response (TIS ≥60): 46% (30 mg) vs 26% (placebo), P = .0126.
- Steroid independence: 42% of brepocitinib patients off corticosteroids at week 52 vs 23% placebo.
- Skin remission: CDASI ≤5 achieved by 44% (30 mg) vs 21% placebo (P = .006).
- Muscle & function: Significant improvements in DMOMS scores (P = .0014), proximal muscle strength, and disability (P = .0035).
- Safety: Slight increase in serious infections, consistent with other TYK2/JAK1 inhibitors. Importantly,
“There were no deaths, cardiovascular or thromboembolic events during the study,” Fernandez noted.
Clinical Significance
Fernandez emphasized that these findings may represent a turning point in dermatomyositis care, introducing the first novel targeted therapy in decades:
“These data are exciting. There has not been a novel medicine approved for dermatomyositis in decades. An IVIG brand was officially approved by the FDA in 2021, but the reality is that this did not change our practice — we had already been using IVIG and had been able to get it covered by insurance for well over a decade prior to that FDA approval. These new data suggest that brepocitinib could be a novel medicine that, if approved by the FDA, really would change the treatment algorithm.”
Priovant plans to submit a new drug application to the FDA in early 2026. Fernandez added:
“Dermatomyositis can be challenging to treat, and it is not uncommon for patients to be on multiple immunomodulatory medications at the same time to try to get either the skin disease or the muscle disease — or sometimes both — under adequate control. If this drug is approved, it could be an addition that helps us more readily get our patient’s disease under control.”
Source & Disclosures
- Study: Vleugels RA, et al. Efficacy and safety of brepocitinib in patients with active dermatomyositis: Results from a randomized, double-blind, multicenter, placebo-controlled, phase III (VALOR) trial. Presented at the Rheumatologic Dermatology Society annual meeting, Oct. 25, 2025, Chicago.
- Disclosures: Priovant sponsored the VALOR study. Fernandez has served as a research investigator for AstraZeneca, Boehringer Ingelheim, Insight, Pfizer, and Priovant, and received consultant/speaking fees from Amgen, Biogen, Bristol Myers Squibb, EMD Serono, Galderma, and Mallinckrodt.