FDA Approves Itvisma for Spinal Muscular Atrophy Treatment
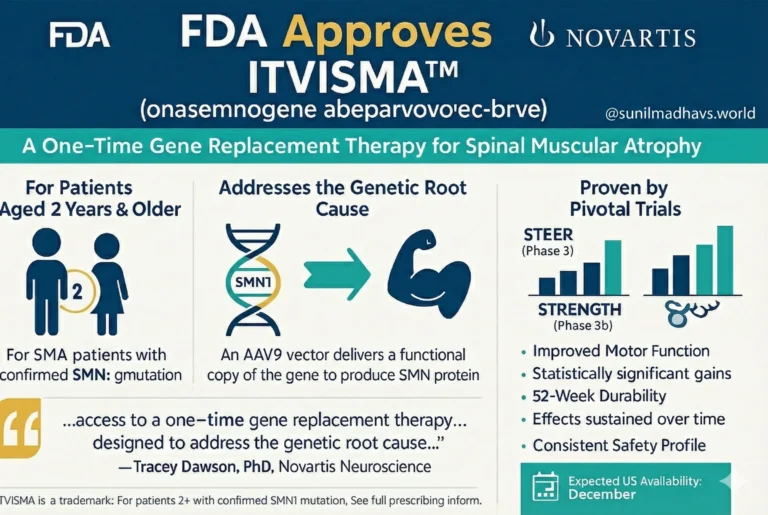
The FDA has granted approval to Novartis for Itvisma (onasemnogene abeparvovec-brve), a new gene replacement therapy designed for patients aged 2 years and older who have been diagnosed with spinal muscular atrophy (SMA) and possess a confirmed mutation of the survival motor neuron 1 (SMN1) gene.
According to the manufacturer, this approval marks a significant milestone, establishing Itvisma as the first and only gene replacement therapy available to treat SMA across virtually the entire lifespan of the patient.
Clinical Evidence and Efficacy
The regulatory approval was driven by data derived from two key studies: the Phase 3 STEER clinical trial and the supporting open-label Phase 3b STRENGTH study.
Outcomes: In these trials, the therapy demonstrated statistically significant improvements in motor function as well as the stabilization of motor abilities.
Durability: These positive effects were sustained over a follow-up period of 52 weeks.
Safety: Novartis reported that the safety profile of the drug remained consistent across both studies.
Tracey Dawson, PhD, the senior vice president and U.S. therapeutic area head of neuroscience at Novartis, highlighted the necessity of this approval for the patient community:
“We believe every patient with SMA deserves access to transformative treatment, regardless of age or disease severity. Many older children, teens and adults continue to face unmet needs and disease progression.”
Understanding the Mechanism and Disease
SMA is a neuromuscular condition caused by a missing or mutated SMN1 gene. This gene is critical because it produces the majority of the SMN protein needed to sustain essential muscle functions, such as movement, swallowing, and breathing.
While the SMN2 gene also produces SMN protein, it creates a much smaller portion of functional protein. Consequently, disease severity often correlates with SMN2 copy numbers—patients with more copies generally experience a less severe form of the condition than those with fewer copies.
Itvisma functions as an adeno-associated virus 9 (AAV9) gene replacement therapy. By targeting the underlying genetic issue, it offers a distinct advantage over chronic management options.
With the approval of Itvisma, this broad range of patients with SMA can have access to a one-time gene replacement therapy that is uniquely designed to address the genetic root cause of their disease, offering the potential to reduce the need for chronic treatment and improve motor function,” Dawson stated.
Dosage and Availability
Administration: Itvisma is administered via a one-time fixed intrathecal dose. Uniquely, this dosage does not require adjustments based on the patient’s body weight or age.
Market Release: The therapy is expected to become available in the United States starting in December.


