Key Points
- Monthly injections of olezarsen lowered triglyceride levels in adults with high cardiovascular (CV) risk.
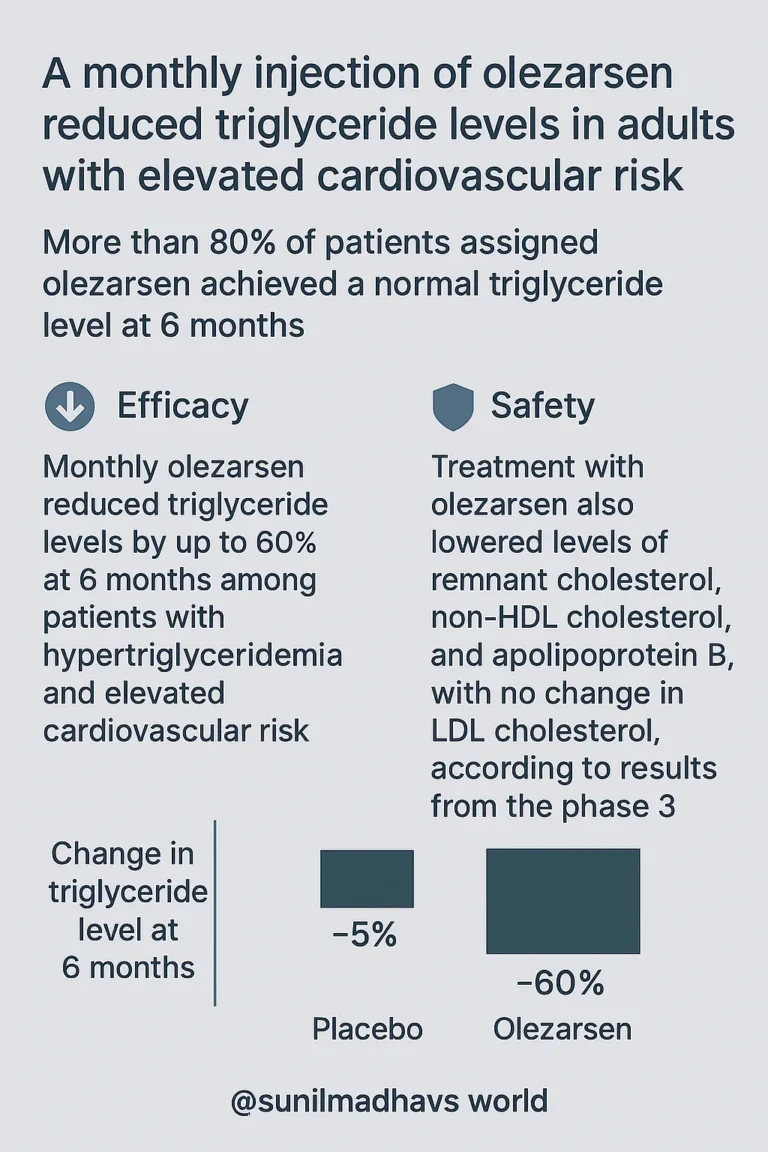
- Over 80% of patients on olezarsen achieved normal triglyceride levels within 6 months.
- Treatment reduced triglycerides by up to 60% at 6 months in patients with moderate hypertriglyceridemia and CV risk.
- Olezarsen also lowered remnant cholesterol, non-HDL cholesterol, and apolipoprotein B (ApoB), but did not affect LDL cholesterol, based on findings from the phase 3 Essence-TIMI 73b trial.
Trial Findings & Expert Insights
At the European Society of Cardiology Congress, Dr. Brian A. Bergmark from the TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School, highlighted that olezarsen’s triglyceride-lowering effect exceeded what current standard therapies can achieve.
He noted that elevated triglycerides are a well-known risk factor for atherosclerotic cardiovascular disease (ASCVD), yet effective treatments remain limited. Olezarsen, an antisense oligonucleotide, works by targeting the messenger RNA of apolipoprotein C-III, a protein involved in triglyceride metabolism.
In 2024, the FDA approved olezarsen (marketed as Tryngolza) for treating familial chylomicronemia syndrome (FCS), a rare genetic condition. However, its broader use in patients with more common forms of hypertriglyceridemia and increased CV risk had not been established — leading to this trial.
Study Design
The phase 3 Essence-TIMI 73b trial was:
- Double-blind, randomized, and placebo-controlled.
- Included patients with:
- Moderate hypertriglyceridemia (150–499 mg/dL) with existing ASCVD or type 2 diabetes (age ≥55), or
- Severe hypertriglyceridemia (≥500 mg/dL).
- Median age of participants: 64 years, with 40% women.
- Median baseline triglyceride level: 238.5 mg/dL.
A total of 1,349 patients were randomized to receive:
- Olezarsen 50 mg,
- Olezarsen 80 mg, or
- Placebo.
The drug was given as a subcutaneous injection every 4 weeks for 12 months.
Results
- Primary outcome: Change in triglyceride levels at 6 months compared to placebo.
- Olezarsen 50 mg → –58.4% reduction.
- Olezarsen 80 mg → –60.6% reduction.
- Both were highly significant (P < .001).
- Normalization of triglyceride levels (<150 mg/dL):
- Placebo: 12.5% of patients.
- Olezarsen 50 mg: 85% of patients.
- Olezarsen 80 mg: 88.7% of patients.
- Other lipid changes with olezarsen:
- Non-HDL cholesterol: ↓ up to 22%
- VLDL cholesterol: ↓ 57%
- Remnant cholesterol: ↓ 68%
- Apolipoprotein B (ApoB): ↓ 15%
- LDL cholesterol: no change
Safety Profile
- Adverse event rates were similar between olezarsen and placebo groups:
- Overall AEs: 72.8–76.7% (olezarsen) vs. 72.1% (placebo).
- Serious AEs: 9.4–13.6% (olezarsen) vs. 11.4% (placebo).
- More injection-site reactions were observed in the treatment arms.
- Rare increases in liver enzymes occurred but were consistent across groups.
- No major safety concerns were identified.
Next Steps
Researchers emphasized that while olezarsen produced significant biochemical improvements, whether these translate into clinical cardiovascular benefits (such as fewer heart attacks or strokes) remains to be proven in a dedicated outcomes trial.
Disclosures
- The Essence-TIMI 73b trial was sponsored by Ionis Pharmaceuticals.
- Dr. Bergmark reported research grants via his institution from multiple companies including Abbott Vascular, Amgen, AstraZeneca, Ionis, Pfizer, Philips, among others, and has served as a consultant to various medical device and pharmaceutical firms.
✅ In short: Olezarsen dramatically lowers triglycerides and improves related lipid markers with a favorable safety profile. While promising, further research is needed to confirm long-term cardiovascular outcome benefits.


