Baxdrostat Shows Promise for Patients With Uncontrolled or Resistant Hypertension
A new phase 3 clinical trial has demonstrated that baxdrostat, an aldosterone synthase inhibitor developed by AstraZeneca, significantly lowers blood pressure in patients with uncontrolled or resistant hypertension.
The results, presented at the European Society of Cardiology Congress and simultaneously published in The New England Journal of Medicine, show that baxdrostat, when added to standard antihypertensive therapy, substantially reduced seated systolic blood pressure (BP) after 12 weeks of treatment.
Why This Matters
Hypertension continues to affect more than one billion people worldwide, and despite the availability of multiple medications, a large proportion of patients remain uncontrolled. This leaves them at heightened risk of heart attack, stroke, heart failure, kidney disease, and dementia.
Dr. Jenifer M. Brown, a cardiologist at Brigham and Women’s Hospital, explained that aldosterone, a hormone involved in sodium and fluid retention, plays a central role in driving high blood pressure and related cardiovascular outcomes. Baxdrostat, as part of a new drug class targeting aldosterone production, aims to address this underlying mechanism.
The BaxHTN Trial
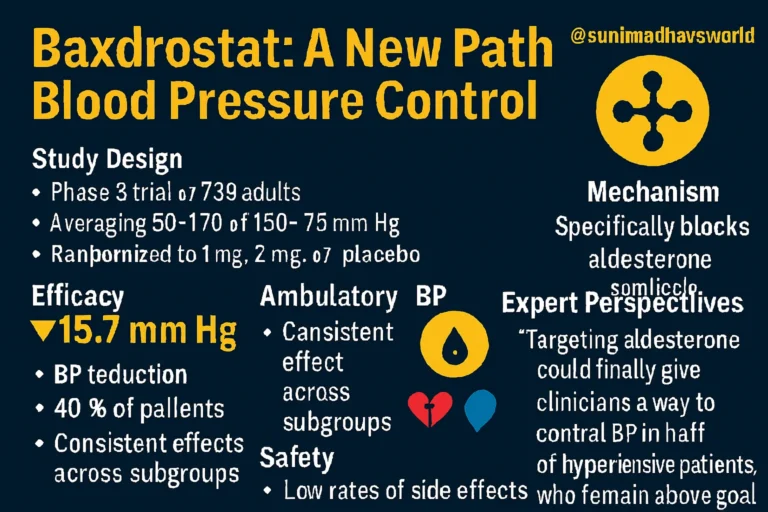
- Participants: 796 adults with systolic BP between 140–170 mm Hg, despite taking at least two antihypertensive drugs (uncontrolled hypertension) or three or more including a diuretic (resistant hypertension).
- Demographics: Average age was around 60 years; most participants were men and white.
- Design: After a 2-week placebo run-in, patients were randomized to receive baxdrostat 1 mg, baxdrostat 2 mg, or placebo once daily for 12 weeks.
- Primary Endpoint: Change in seated systolic BP at week 12.
Efficacy Findings
- Mean reduction in seated systolic BP at 12 weeks:
- –14.5 mm Hg with baxdrostat 1 mg
- –15.7 mm Hg with baxdrostat 2 mg
- –5.8 mm Hg with placebo
- Placebo-adjusted reductions were:
- –8.7 mm Hg for baxdrostat 1 mg
- –9.8 mm Hg for baxdrostat 2 mg (both P < .001).
- BP Control Achieved: About 40% of patients on baxdrostat reached systolic BP < 130 mm Hg, compared with only 18.7% in the placebo group.
An exploratory substudy with 24-hour ambulatory BP monitoring confirmed these findings:
- Baxdrostat 1 mg lowered BP by –13.7 mm Hg
- Baxdrostat 2 mg lowered BP by –16 mm Hg
- Placebo group saw a slight increase of +1 mm Hg
- Night-time systolic BP was an average of 11.7 mm Hg lower with baxdrostat compared with placebo.
Professor Bryan Williams of University College London emphasized that this drug could be a “game changer”, noting that despite best efforts, nearly half of patients remain uncontrolled with existing therapies. Baxdrostat directly targets a core mechanism of hypertension, potentially reducing risks of future cardiovascular and kidney complications.
Safety Profile
- Adverse events (AEs):
- Baxdrostat 1 mg: 47.3%
- Baxdrostat 2 mg: 44.7%
- Placebo: 41.3%
- Most common AEs:
- Elevated potassium (hyperkalemia)
- Low sodium levels (hyponatremia), usually appearing within 2 weeks and then stabilizing.
- Serious lab changes:
- Potassium >6 mmol/L in 2.3% (1 mg), 3% (2 mg), and 0.4% (placebo).
- Mild hyponatremia in 19.1% (1 mg), 22.8% (2 mg), and 7% (placebo).
- Only a small percentage required intervention or treatment discontinuation.
According to Dr. Brown, the adverse events were expected, generally manageable, and aligned with the drug’s mechanism of action.
Conclusion
After decades without a new class of antihypertensive drugs, baxdrostat represents an exciting development. By specifically targeting aldosterone synthesis, it not only lowers blood pressure effectively but may also reduce long-term risks of cardiovascular and kidney disease.

