Key Details:
– Drug: Vizz™ (aceclidine ophthalmic solution 1.44%)
– Indication: Treatment of presbyopia in adults.
– Availability: Expected Q4 2024 (samples in October).
Mechanism of Action
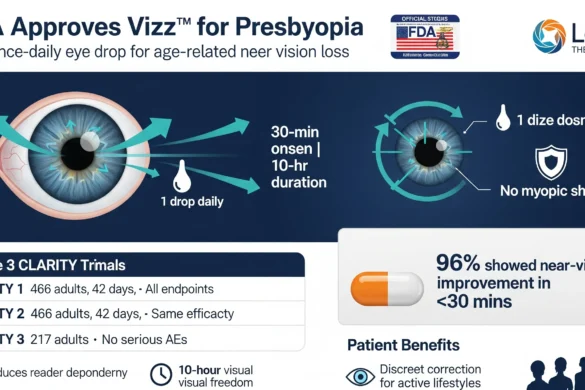
Vizz is a once-daily eye drop that:
1. Contracts the iris sphincter muscle to create a pinhole effect.
2. Extends depth of focus to improve near vision.
3. Avoids myopic shift (unlike some corrective lenses).
Clinical Validation: Phase 3 CLARITY Trials
| Trial | Participants | Duration | Key Outcomes |
| CLARITY 1 | 466 adults | 42 days |
✅ Met all primary/secondary endpoints<br>✅ Near vision improvement within 30 mins<br>✅ Effects lasted ≤10 hours |
| CLARITY 2 | 466 adults | 42 days |
✅ Identical efficacy to CLARITY 1 |
| CLARITY 3 | 217 adults | 6 months |
✅ Strong long-term safety profile<br>⛔ No serious treatment-related adverse events |
Commercial & Patient Impact
– Patient Benefits:
– Reduces dependency on reading glasses.
– Provides “visual freedom” for active lifestyles (≤10 hours).
– Target Users:
– Contact lens wearers seeking glasses-free near vision.
– Post-refractive surgery patients.
– Active individuals prioritizing discreet correction.
– Professional Value:
– Offers eye care providers a complementary treatment option to existing solutions.
Executive Insight
> Eef Schimmelpennink (CEO, Lenz Therapeutics):
> “Vizz delivers a convenient, transformative solution that lets presbyopes stay in the moment. It addresses unmet needs across diverse patient segments seeking independence from readers.”
Key Takeaways
1. FDA approval grounded in robust Phase 3 data (safety + efficacy).
2. Rapid action: Near vision improvement in 30 minutes.
3. Sustained effect: Up to 10 hours per dose.
4. Expanded presbyopia management: Non-invasive alternative to glasses/surgery.


